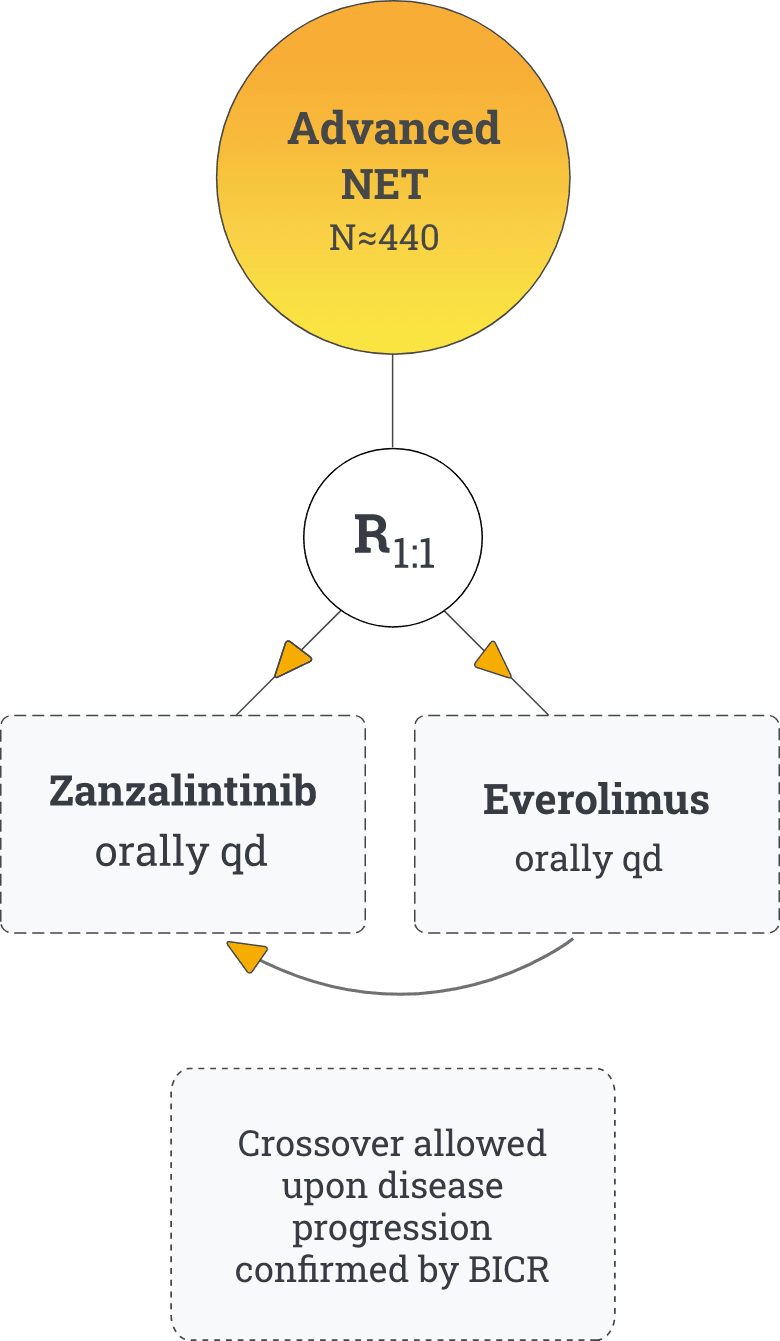
Study Overview
For the phase 2 stage of the trial, approximately 120 eligible patients with unresectable, locally advanced or metastatic NET will be randomized in a 1:1 ratio to either zanzalintinib or everolimus. If the phase 2 analysis supports trial continuation to phase 3, an additional 320 patients will be randomized for a total of approximately 440 patients in this trial. The primary objective of the trial is to evaluate PFS by BICR of zanzalintinib vs everolimus.
Stratification Factors
-
Primary tumor site: pNET vs epNET
-
Prior PRRT: yes vs no
-
Tumor grade: Grade 1 or typical carcinoid vs Grades 2 or 3 or atypical carcinoid